Background: Steroid-refractory (SR) gastrointestinal acute GVHD (GI aGVHD) is a serious complication after allogeneic hematopoietic transplantation (allo-HCT), and subsequent lines of treatment following steroid failure are often ineffective. The JAK 1/2 inhibitor ruxolitinib is the only FDA-approved treatment for SR aGVHD, and patients for whom ruxolitinib is not effective or not tolerated have limited therapeutic options and high mortality. Interleukin-22 (IL-22) is a tissue-protective IL-10-family cytokine that has been shown to support intestinal mucosa after damage, directly signaling to the intestinal epithelium and promoting its survival, regeneration, and production of innate antimicrobial molecules. F-652, a recombinant human (rh)IL-22 fusion protein composed of an IL-22 dimer and IgG2 Fc fragment, has recently been studied in a phase 2 trial for patients with newly diagnosed lower GI aGVHD, demonstrating a promising day 28 treatment response rate and improved gut microbial health. Given this, we examined F-652 administration in 3 patients with severe SR GI aGVHD.
Methods: Three patients with hematologic malignancies underwent MUD allo-HCT with tacrolimus and methotrexate GVHD prophylaxis ( Table). The patients were initially treated for GI aGVHD with methylprednisolone at a dose of 2 mg/kg/day without response. All patients presented with or progressed to stage 4 gut aGVHD and failed several lines of therapy, including ruxolitinib. All patients also had multiple episodes of bacteremia and at least some degree of GVHD-associated GI bleeding. F-652 treatment was initiated under single-patient-use authorization as the 5 th-7 th line of therapy and was administered at a dose of 45 mcg/kg IV weekly. In vitro cultures suggested that rho kinase inhibition did not interfere with trophic effects of IL-22 on intestinal epithelium, so patients were permitted to continue belumosidil upon F-652 initiation. While there was concern that JAK inhibition could compromise the activity of IL-22, continuation of ruxolitinib was considered on a case-by-case basis. C-reactive protein (CRP) is an acute phase reactant induced by IL-22, and circulating CRP levels served as a pharmacodynamic marker of in vivo biologic activity of F-652.
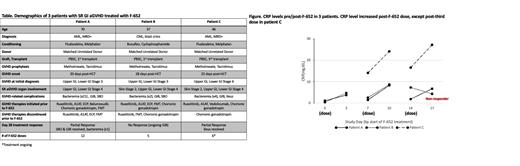
Results: Consistent CRP elevations were observed 3 days after F-652 dosing in patients A and C, both of whom demonstrated a reduction in GVHD symptoms following F-652 treatment. These CRP elevations occurred despite concurrent use of belumosidil in Patient A and ruxolitinib in Patient C. CRP levels increased in Patient B after the first two doses, but not the third ( Figure). By day 28 following F-652 initiation, 2-of-3 patients (A and C) achieved a partial response, with decreased stool output and resolution of GI bleeding. Patient A had experienced 21 episodes of bacteremia prior to initiating rhIL-22 therapy but continued to demonstrate a partial response at day 56 and resolutiuon of bacteremias. Further improvement during the continued treatment (12 total doses) allowed for hospital discharge after 14 months of hospitalization. Patient B received 4 failed lines of therapy beyond ruxolitinib and was experiencing GI bleeding necessitating frequent transfusions prior to F-652 initiation. The symptoms persisted following F-652 treatment, and the patient passed at day 149 post-HCT. Patient C continues on active F-652 treatment and remains in a partial response as of this submission (> 50 days after treatment initiation). Overall, F-652 treatment has been well tolerated, and no significant adverse events attributable to the drug were observed.
Conclusions: This is the first report of IL-22 therapy in SR aGVHD. Treatment was well tolerated, and 2-of-3 patients achieved a response following rhIL-22 administration after failing multiple previous therapies. Whereas most treatments for GVHD target immune cells and deepen immunosuppression, IL-22 is understood to act directly upon epithelial cells where it can support tissue recovery, improve barrier function, and promote innate antimicrobial immunity. CRP elevations post-treatment suggest that IL-22 administration may be able to exert these effects even with concurrent use of a JAK inhibitor. Our findings support further development of this approach and provide proof-of-concept for the use of tissue-supportive strategies to enhance the recovery of damaged gastrointestinal mucosa in advanced lower GI aGVHD.
OffLabel Disclosure:
Gyurkocza:Actinium Pharmaceuticals, Inc: Research Funding. Giralt:Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding; Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees. Perales:VectivBio AG: Consultancy, Honoraria; DSMB: Other; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Exevir: Consultancy, Honoraria; Miltenyi Biotec: Honoraria; MorphoSys: Consultancy, Honoraria; Kite: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Equillium: Consultancy, Honoraria; Vor Biopharma: Consultancy, Honoraria; Servier: Other; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Allogene: Research Funding; Incyte: Consultancy, Honoraria, Research Funding; NexImmune: Consultancy, Current equity holder in publicly-traded company; Sellas Life Sciences: Consultancy; Medigene: Consultancy, Other; Syncopation: Honoraria; Cidara Therapeutics: Consultancy, Other; Caribou: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; Astellas: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Allovir: Consultancy; Adicet: Honoraria; Celgene: Honoraria; Karyopharm: Consultancy, Honoraria; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria. Chen:Evive Biotechnology: Current Employment. Li:Evive Biotechnology: Current Employment. Peled:Postbiotics Plus Research: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; MaaT Pharma: Consultancy; DaVolterra: Consultancy; CSL Behring: Consultancy; Seres Therapeutics: Other: travel reimbursement, intellectual property fee, Research Funding. van den Brink:Seres Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: IP licensing , Research Funding; Da Volterra: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Thymofox: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Lygenesis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Ceramedix: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Notch Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; DKMS (a non-profit organization): Membership on an entity's Board of Directors or advisory committees; Pluto Immunotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Rheos Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Frazier Healthcare Partners: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Vor Biopharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Other: IP licensing; Wolters Kluwer: Patents & Royalties. Hanash:Evive Biotechnology: Other: intellectual property related to Interleukin-22; ASTCT: Membership on an entity's Board of Directors or advisory committees. Ponce:Ceramedix: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kadmon/Sanofi Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Evive Biotechnology: Membership on an entity's Board of Directors or advisory committees.
recombinant human IL-22 or F-652 for the purpose of treating acute gastrointestinal GVHD